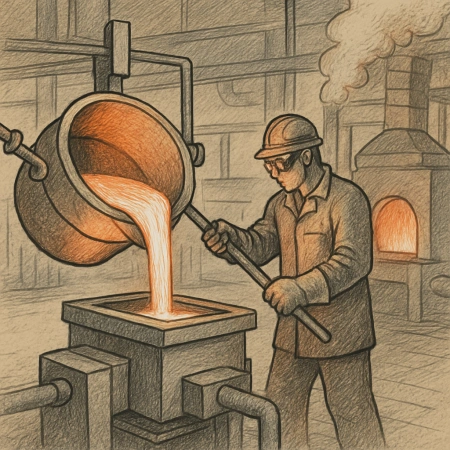
Carbon anodes play a critical role in various industrial processes, especially in aluminum production. They serve as the primary conductor of electricity, enabling the electrochemical reactions necessary to produce aluminum. However, despite their importance, carbon anodes need regular replacement. This article explores why that is the case and the implications of their periodic renewal.
Carbon anodes are large blocks of carbon used in the electrolysis process of aluminum production. They are typically made from petroleum coke and pitch, which are molded and baked to form a solid structure. In the electrolytic cell, the carbon anode conducts electricity into the molten electrolyte, facilitating the reduction of aluminum ions to aluminum metal.
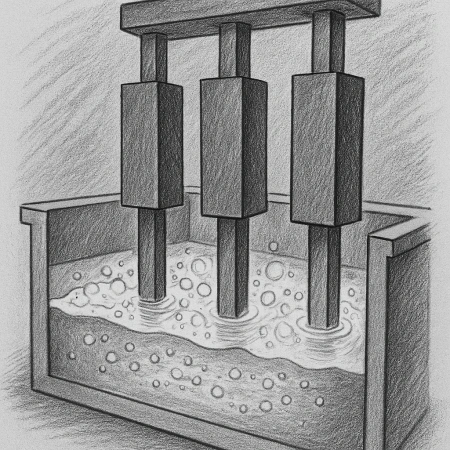
During electrolysis, carbon anodes participate in the chemical reaction that separates aluminum from its ore. As the electric current passes through the anode, it reacts with the oxygen in the alumina (aluminum oxide), forming carbon dioxide gas. This reaction is crucial for the production of aluminum but also leads to the gradual consumption of the anode itself.
One of the primary reasons carbon anodes need to be replaced regularly is due to their consumption during the electrolysis process. The reaction between carbon and oxygen produces carbon dioxide, gradually wearing down the anode. This consumption is an inherent part of the aluminum production process and cannot be avoided.
Apart from chemical consumption, carbon anodes also experience physical wear and tear. The high temperatures and harsh conditions inside the electrolytic cells can cause the anodes to crack or deteriorate over time. This structural degradation can affect their performance and necessitate replacement to maintain efficiency in the production process.
As carbon anodes wear down, their efficiency in conducting electricity can diminish. This can lead to increased energy consumption and reduced productivity. Moreover, worn-out anodes can introduce impurities into the aluminum, affecting its quality. Regular replacement ensures the process remains efficient and the aluminum produced meets quality standards.
In industrial settings, the replacement of carbon anodes is typically scheduled as part of routine maintenance. Operators monitor the anodes' condition and replace them before they become too degraded to function effectively. This proactive approach helps minimize disruptions and maintain continuous production.
Replacing carbon anodes is not without its challenges. The process involves halting production, which can be costly. Additionally, handling and installing new anodes require specialized equipment and skilled personnel. These factors contribute to the overall cost of aluminum production.
Researchers are continually exploring ways to improve the longevity and efficiency of carbon anodes. Innovations in materials and production techniques aim to reduce anode consumption and extend their lifespan. For instance, the development of inert anodes, which do not react with oxygen, holds promise for reducing the frequency of replacements.
The regular replacement of carbon anodes also has environmental implications. The consumption of carbon contributes to greenhouse gas emissions, primarily in the form of carbon dioxide. Efforts to develop more sustainable anode technologies are driven by the need to reduce the industry's carbon footprint.
In summary, the regular replacement of carbon anodes is a necessary aspect of aluminum production. Their consumption during electrolysis and susceptibility to wear and tear necessitate periodic renewal to ensure efficiency and product quality. While this process presents challenges, ongoing research and innovations in anode technology offer hope for more sustainable and cost-effective solutions in the future.
Understanding the reasons behind the frequent replacement of carbon anodes highlights the complex nature of industrial processes and the continuous efforts to optimize them. As technology advances, the aluminum industry may find new ways to enhance efficiency while minimizing environmental impact, ensuring a more sustainable future.