Every aluminum smelter relies on one essential component: the carbon anode block. These massive blocks act as conductors, transferring electric current through the electrolytic cell during aluminum production. But have you ever wondered how ordinary carbon-based materials like petroleum coke are transformed into highly conductive, high-strength anodes?
This transformation is not just engineering — it’s chemistry at work.
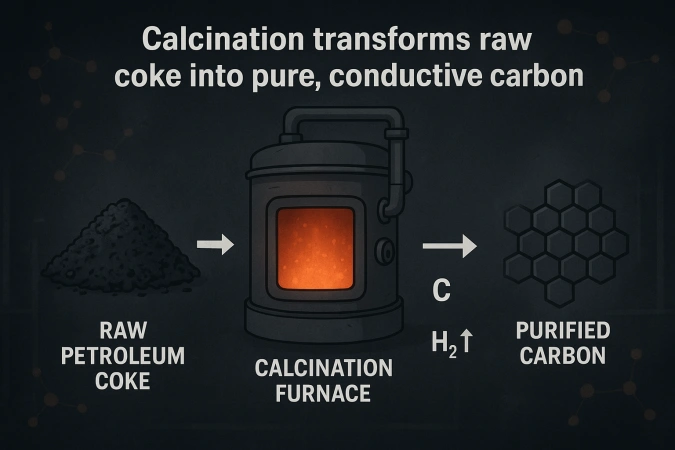
The journey begins with petroleum coke (or sometimes pitch coke), a byproduct of oil refining. Chemically, coke consists primarily of carbon atoms but also contains traces of sulfur, nitrogen, hydrogen, and metals. To transform it into an anode-grade material, impurities must first be reduced through calcination.
During calcination, coke is heated to 1200–1400°C in a rotary kiln or shaft furnace. This drives off volatile matter, removes moisture, and rearranges the carbon atoms into a more ordered, graphitic-like structure.
The result is denser, purer carbon with improved electrical conductivity — the first step in turning coke into a reliable conductor.
Chemistry highlight:
The carbonization reaction CₓHᵧ → C + H₂↑ releases hydrogen and hydrocarbons, while simultaneously increasing the carbon’s crystallinity.
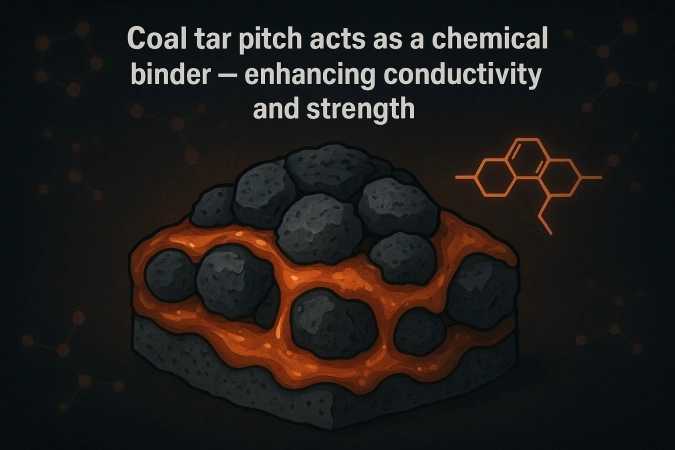
While coke provides the backbone, coal tar pitch acts as the chemical glue. Pitch is a complex mixture of aromatic hydrocarbons derived from coal distillation, rich in carbon and possessing thermoplastic properties.
When heated to around 150–200°C, pitch softens and coats the coke particles. Upon cooling and subsequent baking, it undergoes polycondensation reactions that form a solid, carbon-rich binder phase.
This process chemically integrates the coke and pitch, creating a homogeneous material with both mechanical strength and electrical connectivity. The pitch’s aromatic rings promote carbonization during baking, contributing additional conductive carbon.
Key reaction:
C₁₀H₈ (naphthalene derivatives in pitch) → polyaromatic carbon network + volatiles (CH₄, CO, CO₂)
Once formed into green anode blocks, the mixture is baked at 1000–1200°C in large ring furnaces. This stage initiates the critical carbonization reactions:
Hydrogen, oxygen, and nitrogen compounds decompose;
The pitch converts into additional solid carbon;
Pores and microchannels form, allowing gases to escape.
Chemically, this step increases carbon purity and crystalline order, which directly enhances the block’s electrical conductivity. The final baked anode has a carbon content exceeding 98%, with resistivity typically below 55 µΩ·m.

The chemistry of carbonization bridges organic molecules and crystalline carbon — a transformation from hydrocarbon to pure conductor.
In the electrolytic cell, the baked anode block faces extreme chemical conditions. It reacts with oxygen ions from the alumina bath:
C+2O2−→CO2+4e−C + 2O^{2−} → CO₂ + 4e^−C+2O2−→CO2+4e−
This reaction releases carbon dioxide while freeing electrons that participate in the electrolysis of alumina (Al₂O₃). Thus, the carbon anode is both a chemical reactant and electrical conductor — a consumable yet vital part of the aluminum-making process.
Chemistry in motion: Every electron that produces aluminum originates from carbon oxidation.
The chemistry of carbon anodes is evolving. Researchers are exploring:
Low-sulfur cokes to reduce SO₂ emissions;
Modified pitches with better carbon yields;
Inert anodes made from ceramics or composites to eliminate CO₂ release.
While full decarbonization remains challenging, advances in anode chemistry are paving the way for cleaner and more efficient smelting.
Transforming coke into a conductor is a story of precise chemistry — where hydrocarbons are purified, rearranged, and reborn as crystalline carbon.
Every stage, from calcination to carbonization, is a chemical dance that defines the strength, conductivity, and lifespan of the final anode block.
In the world of industrial electrochemistry, carbon is not just fuel — it’s the conductor of innovation.
Inside the Carbon Anode Block: Understanding the Science Behind Conductivity and Strength
Why Bulk Density Matters: The Hidden Key to Anode Efficiency