In aluminum smelting, carbon anode blocks serve as the heart of the electrolysis process, conducting massive electrical currents while enduring extreme heat and chemical reactions.
But what gives these carbon blocks their remarkable conductivity and mechanical strength?
The answer lies inside their structure — in the intricate world of carbon atoms, crystalline lattices, and engineered porosity.
This article explores the science behind conductivity and strength in carbon anode blocks, revealing how chemistry and microstructure come together to create a truly high-performance material.
Carbon is unlike any other element in the periodic table.
It can bond in multiple configurations — sp² (graphitic), sp³ (diamond-like), and amorphous — giving rise to a range of physical properties.
In carbon anode blocks, the dominant structure is graphitic carbon, characterized by planar hexagonal layers of carbon atoms (graphene sheets). These layers allow electrons to move freely, resulting in excellent electrical conductivity.
Key concept:
The more aligned and ordered these graphitic layers are, the lower the electrical resistivity and the higher the overall conductivity of the block.
Inside the anode, the microstructure consists of:
Coke particles — providing the conductive framework
Pitch-derived carbon — acting as the binder phase
Pores and microcracks — formed during baking
Electron flow primarily occurs through the solid carbon matrix. However, the interfaces between particles and pores can scatter or block electrons.
That’s why controlling particle packing, binder distribution, and pore size is essential to achieving high conductivity.
Did you know?
Even a 5% increase in porosity can reduce conductivity by more than 10%, because electrons must travel longer and less direct paths.
While conductivity depends on the carbon’s crystalline structure, mechanical strength is governed by:
Particle bonding (how well coke and pitch fuse during baking)
Binder carbon content (higher pitch content = stronger bonding)
Pore distribution (uniform, fine pores resist cracking)
During baking at around 1100–1200°C, pitch transforms into solid carbon through carbonization reactions, effectively “welding” particles together.
This process gives the block both compressive strength and thermal stability, allowing it to withstand the stress of electrolysis and gas evolution.
Mechanical insight:
Optimal baking conditions produce an anode with compressive strength above 35 MPa and minimal internal stresses.
Conductivity and strength don’t always increase together.
For example:
Higher density (fewer pores) improves conductivity, but excessive compaction can trap volatiles, weakening the block.
More binder improves bonding but may lead to uneven carbonization and internal cracking.
Achieving the right balance requires precise control over raw material quality, mixing ratio, forming pressure, and baking temperature — a blend of chemistry and engineering.
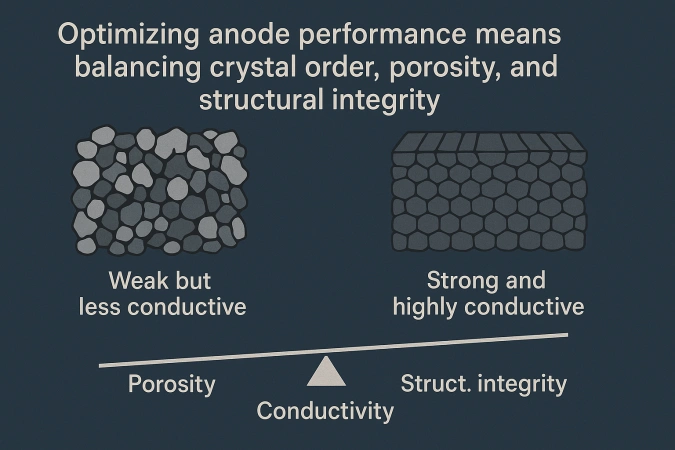
Balance is key: The most efficient anodes are those that find the sweet spot between crystal alignment, porosity, and structural integrity.
Once installed in the electrolytic cell, the anode continues to evolve.
As it reacts with oxygen ions, it slowly oxidizes, creating a thin layer of CO₂ and CO gas release.
At the same time, localized heating can cause recrystallization, slightly improving conductivity over time until the anode is consumed.
This dynamic environment highlights how the anode’s microstructure and chemistry determine not only its initial performance but also its lifespan and efficiency in operation.
Understanding what happens inside the carbon anode block reveals the elegant interplay between atomic structure, microstructure, and manufacturing chemistry.
Every layer, pore, and bond contributes to the delicate balance of conductivity and strength that makes modern aluminum production possible.
In essence, each carbon block is more than a passive conductor — it’s a carefully engineered material, designed to turn chemistry into performance.